Can we measure the brain’s exaggerated response to pain and sensory input?
A team of researchers in Colorado have demonstrated the effectiveness of utilizing neuroimaging-based tools to help validate pain symptoms and gauge treatment response in patients with fibromyalgia (FM). Such techniques have the potential to benefit Lyme disease patients by helping to determine the effectiveness of various therapies for each patient.
Studies have found that patients with Lyme disease (LD) experience “exaggerated responses to pain and non-painful stimuli” despite antibiotic treatment. [1, 2] Lopez-Sola and colleagues describe those same responses in patients with fibromyalgia (FM), a condition associated with widespread musculoskeletal pain and tenderness accompanied by fatigue, cognitive, emotional and sleep-related symptoms. [3]
“In addition to pain-related changes,” the authors point out that “FM [fibromyalgia] patients show reduced tolerance (augmented unpleasantness) to non-painful sensory stimulation (visual, auditory, olfactory and tactile), along with abnormal brain processing of non-painful sensory stimuli.” [3] Their findings were recently reported in the journal PAIN, published by the International Association for the Study of Pain.

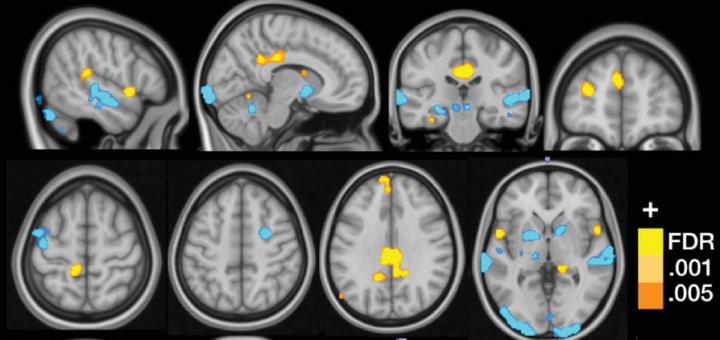
An MRI image showing the multivariate brain pattern that predicts fibromyalgia status on the basis of brain activation during multisensory stimulation. Credit: Cognitive and Affective Control Laboratory / University of Colorado Boulder.
Lopez-Sola’s team used Functional Magnetic Resonance Imaging (fMRI) to demonstrate an exaggerated response to pain and non-painful stimuli in fibromyalgia patients. fMRI has previously been used to measure regional, time-varying changes in the brain’s metabolism as a biomarker for disease and to monitor therapy, or for studying pharmacological efficacy. [4] PET and SPECT scans are also used to measure brain function.
The team exposed 37 fibromyalgia patients and 35 control patients to painful pressure and non-painful visual, auditory, and tactile stimuli. “When the people with fibromyalgia were exposed to the painful stimuli, they had greater Neurologic Pain Signature (NPS) responses than those without the condition.”
Greater responses were also described for non-painful visual, auditory, and tactile stimuli. “In fibromyalgia, the misfiring and irregular engagement of different parts of the brain to process normal sensory stimuli like light, sound, pressure, temperature and odor, results in pain, flu-like sensations or other symptoms. This extra brain work can be exhausting,” explains Jan Chambers, founder of the National Fibromyalgia & Chronic Pain Association. [5]
“The potential for brain measures like the ones we developed here is that they can tell us something about the particular brain abnormalities that drive an individual’s suffering,” states Wager. [6] The fMRI can provide clinicians with insight into the neural pathology underlying fibromyalgia pain symptoms. According to Lopez-Sola, “The novelty of this study is that it provides potential neuroimaging-based tools that can be used with new patients to inform about the degree of certain neural pathology underlying their pain symptoms.” [5]
The fMRI results may also help predict treatment responses in fibromyalgia by establishing “a framework for assessing therapeutic mechanisms and predicting treatment response at the individual level,” states Lopez-Sola.
“The brain changes due to pain consisted of augmented responses in sensory integration (insula/operculum) and self-referential (e.g., medial prefrontal) regions in fibromyalgia, and reduced responses in the lateral frontal cortex,” describes Lopez-Sola. “The brain changes due to non-painful sensory stimulation consisted of augmented responses in insula/operculum, posterior cingulate, and medial prefrontal regions, and reduced responses in primary/secondary sensory cortices, basal ganglia and cerebellum.”
The authors cautioned that exaggerated responses to pain and non-painful stimuli are not unique to fibromyalgia. “Notably, we do not imply that the signature should differentiate FM from other chronic pain conditions with a sensitization component.”
“Though we take this as a provisional ‘signature’ for FM, we do not imply that these patterns are unique to FM or that they differentiate it from other conditions. Multifocal pain and widespread mechanical hypersensitivity are common features of multiple chronic pain conditions.” [3]
In fact, exaggerated responses to pain and non-painful stimuli, frequently described in Lyme disease, could possibly be measured by an fMRI to help assess therapeutic approaches and predict treatment response for individual Lyme disease patients.
Acute and chronic pain associated with Lyme borreliosis has been characterized as having “qualities of neuropathic pain, that is, radicular, deep aching, or lancinating pain, often worse at night, and associated with both sensory and motor findings,” according to Zimering. “Chronic pain despite adequate antibiotic treatment of neuroborreliosis was reported by a substantial number of patients described by various investigators.” [2]
Lyme disease patients frequently report central sensitization or sensory hyperarousal, as well. “In patients with PTLS [Post-treatment Lyme syndrome], sensory hyperarousal was reported by a majority of patients after acquiring Lyme disease, most often affecting hearing and/or vision.” [1]
“Hypersensitivity to sound may be limited to louder sounds, but, in some individuals, even the volume fluctuations in a normal conversation can be noxious,” according to Batheja from the Department of Psychiatry, Columbia University. [1] “These patients might be seen wearing earplugs or sound-protectors when in situations normally tolerable to others.”
“The individual’s life may be quite altered by this hypersensitivity: wearing sunglasses indoors and avoidance of being outside during daylight, which, in turn, limits the ability to sustain a normal work and social life.” [1]
Hypersensitivity in other sensory modalities, such as olfactory, tactile, gustatory, and temperature have been described, according to Batheja. You can learn more about sensitivity to smell in Lyme disease patients in an All Things Lyme blog “What’s that smell?” [7]
References:
- Batheja, S., et al., Post-treatment lyme syndrome and central sensitization. J Neuropsychiatry Clin Neurosci, 2013. 25(3): p. 176-86.
- Zimering, J.H., et al., Acute and chronic pain associated with Lyme borreliosis: clinical characteristics and pathophysiologic mechanisms. Pain, 2014. 155(8): p. 1435-8.
- Lopez-Sola, M., et al., Towards a neurophysiological signature for fibromyalgia. Pain, 2016.
- Glover, G.H., Overview of functional magnetic resonance imaging. Neurosurg Clin N Am, 2011. 22(2): p. 133-9, vii.
- Researchers Discover ‘Brain Signature’ for Fibromyalgia by Pat Anson in Pain News Network on August 18, 2016 in https://www.painnewsnetwork.org/stories/2016/10/18/researchers-discover-brain-signature-for-fibromyalgia.
- Breakthrough First Step in Brain-Based Fibromyalgia Diagnosis by Caitlyn Fitzpatrick in HCPlive on August 17, 2016
in https://www.hcplive.com/medical-news/breakthrough-first-step-in-brain-based-fibromyalgia-diagnosis. - What is that smell? All Things Lyme Blog by Dr. Daniel Cameron at https://danielcameronmd.com/whats-that-smell/.



Join the Lyme Conversation
(Note: comments are moderated. You will see your comment after it has been reviewed.)