by Daniel J. Cameron, MD MPH
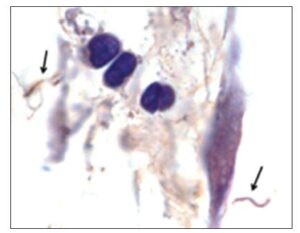
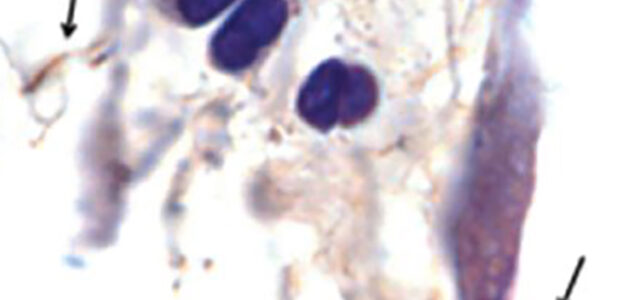
Persistent infection of Borrelia burgdorferi (Bb) has been proven experimentally in Peromyscus mice, laboratory mice, rats, hamsters, gerbils, guinea pigs, dogs and non-human primates. [1-8] Some researchers and clinicians argue, the existence of a persistent Bb infection explains why some Lyme disease patients remain sick even after treatment. According to one study, as many as one-third of patients treated for Lyme disease remain chronically ill. [9]
In a study review published in the Bosnian Journal of Basic Medical Sciences, [10] Dr. Emir Hodzic, of Real-Time PCR Research and Diagnostic Core Facility, School of Veterinary Medicine, University of California, Davis, explores the role ‘persisters’ play in causing chronic Lyme symptoms.
“The main culprit responsible for the tolerance of pathogens to antibiotics is a specialized survivor – a persister,” states Hodzic. [10] Since persisters are non-growing dormant cells, antimicrobials cannot kill them and they cannot be cultured. For antibiotics to work effectively, there must be active target cells to attack.
During the course of infection, Borrelia burgdorferi proliferates, generating ‘attenuated’, or weakened, spirochetes that have lost one or more small plasmids (small DNA molecule within a cell). The attenuated spirochetes remain viable but because of their plasmid loss, they divide slowly. This makes them tolerant of antimicrobials and non-cultivable.
“There is clear scientific evidence that a small, heterogeneous subpopulation of surviving spirochetes shows tolerance to antimicrobial agents and can persist in a host for a prolonged period following therapy,” states Hodzic. [10]
Additionally, persisters do not appear immediately. They emerge months after treatment. Hodzic found that non-cultivable spirochetes reappeared in the tissues of mice 12 months after antibiotic treatment.
“B. burgdorferi is highly prone to plasmid loss [11-13] and therefore, plasmid loss is likely to occur during the course of infection and increase over time. This may explain why treatment success in humans [14,15] and laboratory mice [16,17] appears to be most effective during early infection.” [10]
Persisters also appear in farm and pet animals, insects, rodents and wild animals, including migratory birds. Selection for antimicrobial resistance/tolerance “can occur anywhere antimicrobial agents are present, the environments most notably sewage and surface,” according to Hodzic. [10]
Transmission of antimicrobial resistant/tolerant pathogens of animal origin to humans has been documented for methicillin-resistant S. aureus, E. coli, Salmonella spp., and Campylobacter spp. Patients might run into a persister before being treated.
The presence of persisters may explain why it is sometimes difficult to find an effective antibiotic to treat Lyme disease patients. Hodzic explains that the antibiotic tolerance in persisters is “likely explained by antimicrobial tolerance (in contrast to antibiotic resistance or inadequate antibiotic treatment), in which all classes of antibiotics fail to completely eliminate non-dividing or slowly dividing subpopulations of a broad array of bacteria and fungi.” [10]
Sources:
- Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol, 26(5), 893-895 (1988).
- Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol, 143(3), 959-971 (1993).
- Moody KD, Barthold SW, Terwilliger GA, Beck DS, Hansen GM, Jacoby RO. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg, 42(2), 165-174 (1990).
- Goodman JL, Jurkovich P, Kodner C, Johnson RC. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J Clin Microbiol, 29(5), 894-896 (1991).
- Preac Mursic V, Patsouris E, Wilske B, Reinhardt S, Gross B, Mehraein P. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals. A comparison with histopathological findings in human Lyme disease. Infection, 18(6), 332-341 (1990).
- Sonnesyn SW, Manivel JC, Johnson RC, Goodman JL. A guinea pig model for Lyme disease. Infect Immun, 61(11), 4777-4784 (1993).
- Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol, 35(1), 111-116 (1997).
- Roberts ED, Bohm RP, Jr., Cogswell FB et al. Chronic lyme disease in the rhesus monkey. Lab Invest, 72(2), 146-160 (1995).
- Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther, 1-33 (2014).
- Hodzic E. Lyme Borreliosis: Is there a preexisting (natural) variation in antimicrobial susceptibility among Borrelia burgdorferi strains? Bosn J Basic Med Sci, 15(3), 1-13 (2015).
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A, 97(25), 13865-13870 (2000).
- Biskup UG, Strle F, Ruzic-Sabljic E. Loss of plasmids of Borrelia burgdorferi sensu lato during prolonged in vitro cultivation. Plasmid, 66(1), 1-6 (2011).
- Schwan TG, Burgdorfer W, Garon CF. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun, 56(8), 1831-1836 (1988).
- Wormser GP, Dattwyler RJ, Shapiro ED et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis, 43(9), 1089-1134 (2006).
- Stanek G, Strle F. Lyme borreliosis. Lancet, 362(9396), 1639-1647 (2003).
- Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother, 52(5), 1728-1736 (2008).
- Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother, 54(2), 643-651 (2010).